Rectifier transformer for electrochemical electrolysis
Overview of rectifier transformers for electrochemical electrolysis
Electrochemical rectifier transformers are widely used to electrolyze non-ferrous metals, electrolyze table salt to produce chlor-alkali, and electrolyze water to produce hydrogen and oxygen. It has the following characteristics:
1 The voltage on the valve side is not greater than 1000 V, and the current can be as high as 100 KA.
2, electrolytic loads are connected day and night.
3 The voltage on the valve side is adjusted frequently, and the voltage adjustment range is usually large.
Electrochemistry is the science of studying the charged interface phenomena formed by two types of conductors and the changes that occur on them. The interaction of electricity and chemical reaction can be completed by the battery, or it can be realized by high-voltage electrostatic discharge (such as oxygen is converted into ozone through a silent discharge tube). . Because discharge chemistry has a special name, electrochemistry often refers specifically to "the science of batteries."
Electrochemistry has now formed many branches such as composition electrochemistry, quantum electrochemistry, semiconductor electrochemistry, organic conductor electrochemistry, spectral electrochemistry, and bioelectrochemistry.
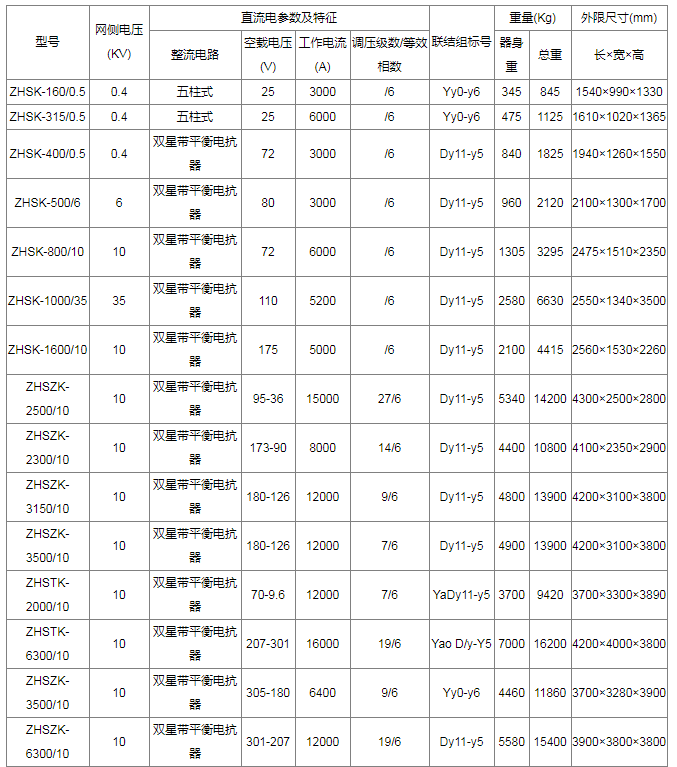
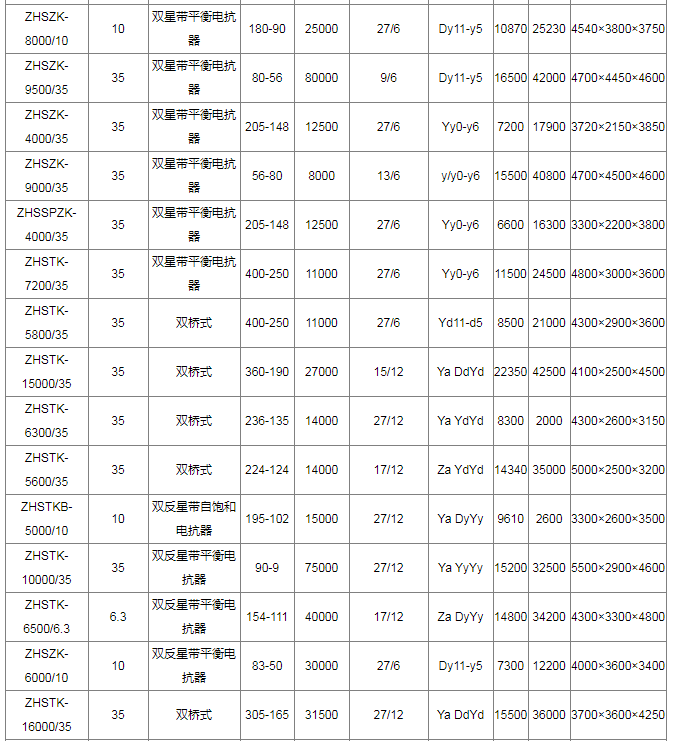
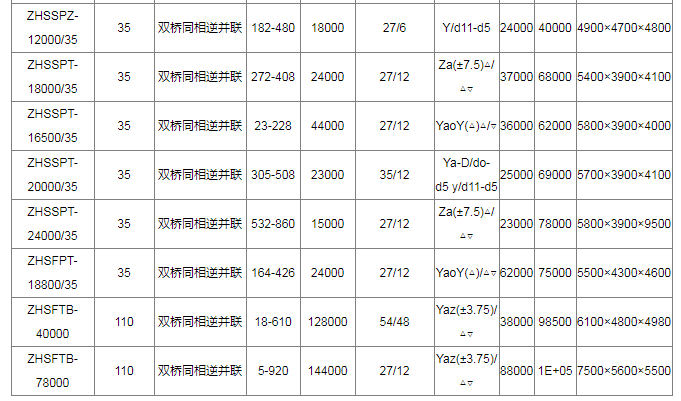
Technical data of electrochemical rectifier transformer